
History
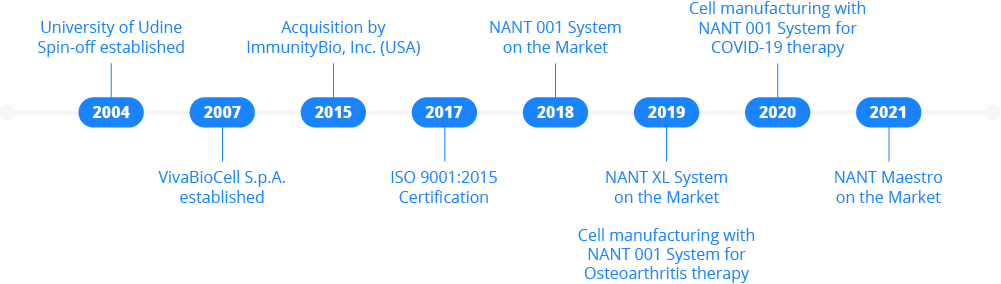
VivaBioCell was born in 2004 as a spin-off of the University of Udine, with the visionary idea of prompting innovation in the field of cell culturing. The company has evolved over years delivering several projects in the field of tissue engineering and cell cultures automation, aimed at the development of new clinical applications.
After the acquisition in 2015 by ImmunityBio Inc., VivaBioCell grew and developed rapidly, reaching the market in 2018 with an innovative bioreactor system, the NANT 001. The challenge to improve and increase the cell therapy manufacturing scale led to the development of the NANT XL System, launched in the market in 2019. In 2021 VivaBioCell released eventually NANT Maestro, a novel software designed to manage and monitor the entire device pool from a single and centralized dashboard.
VivaBioCell Systems are currently utilized in ATMP manufacturing processes for a MSCs-based therapy for osteoarthritis in Europe, and for the production of allogenic BM-Allo.MSC to treat acute respiratory distress syndrome (ARDS) caused by COVID-19 in a clinical Phase Ib study in U.S.A.
Divisions
A cross-functional team of 35 specialists, integrated in 5 divisions, driving the innovation in the field of ATMP manufacturing, and supporting our customers throughout their journey.
Engineering and Manufacturing
From R&D to industrialization and manufacturing. From a concept, all the way towards the design and realization of new products.
Bio and Applications
A team committed to design and optimize new processes on board of our Systems, developing alternative solutions for automated cell culturing.
Service
Constantly supporting our customers, and working together with them to integrate our technology in their cell therapy manufacturing process. Providing support for protocols design, managing training, installation, IQ/OQ and maintenance of our products.
Marketing & Business Development
Managing and building enduring relationships with our Partners and Customers, inspiring the application of our technology to improve the current industry standard of cell therapy manufacturing.
Quality
Application of ISO 9001:2015 to all steps of product development and service provision, continuously improving all processes to ensure the growth of our organization and integrating relevant best practices to our activities in order to meet the needs of our valuable partners, collaborators and customers.
ImmunityBio Inc.
VivaBioCell is fully owned by ImmunityBio Inc. (formerly NantKwest, Inc.) (NASDAQ: IBRX) a subsidiary of NantWorks LLC group (Culver City, California), an ecosystem of companies focused on biotech, healthcare AI, and mobility.
ImmunityBio is focused on developing next-generation cell and immunotherapy solutions intended to eradicate cancer and infectious disease by strengthening the patient’s immune system. Founded in 2014 by Dr. Patrick Soon-Shiong, who has devoted his life to study the mechanisms that enable the immune system to recognize and attack tumors, ImmunityBio is now a late-stage Immunotherapy company with a pipeline to date of over 40 clinical trials across 19 indications in solid and liquid cancers, and infectious diseases.
VivaBioCell and ImmunityBio closely cooperate to improve the current state-of-the-art of cell therapy manufacturing by developing new and innovative tools that will change the way we look at these technologies today.
Partners

Educell is an advanced biotech company and Cell Factory, responsible for the manufacturing with NANT 001 System of AD-MSC used for OA treatment within the EU Grant Project (A.R.T.E.).

The Regenerative Medicine Institute and Centre for Cell Manufacturing Ireland (CCMI)
CCMI is a facility licensed for the manufacture of cell therapy products, where VivaBioCell technology has been validated under conditions fully compliant with GMP.

Centre for Advanced Medical Products (CAMP)
CAMP focuses its activities on the science and technology required to translate ATMP from lab to clinic, with VivaBioCell being the first international partner of this Swedish consortium.

As lead partner of the EU Interreg project Immunocluster, Celica Biomedical is leading the development and follow-on clinical application of a novel cell therapy for the treatment of Triple Negative Breast Cancer.

HTH is a company providing an end-to-end service in the support for the development, manufacture and launch of ATMPs, actively cooperating with VivaBioCell for the assessment and validation of NANT XL System.

Antleron is a company fostering the engineering of advanced therapies, partnering with VivaBioCell with the mission of enabling sustainable pathways for the manufacturing of ATMP.

Cell Factory – Fondazione IRCCS Ca' Granda
The cell factory has provided the GMP validation of AD-MSC manufacturing process (with NANT 001 System) that supported the application for a Phase II Clinical Trial within the A.R.T.E. EU Grant Project.

International Centre for Genetic Engineering and Biotechnology (ICGEB)
ICGEB is an international and Intergovernmental research institution, partner with VivaBioCell in the PREFER Grant Project, where an Advanced Therapy for the treatment of diabetic foot is investigated.

Located in Udine, the University Hospital is involved together with VivaBioCell in different projects having the aim of bringing Advanced Therapies to the clinical stage.

A University Hospital cooperating in the PREFER project and providing clinical expertise in the field of diabetic foot treatment.

A clinic specialized in orthopedic treatments and surgeries, providing AD-MSC therapy for knee OA in the framework of A.R.T.E. Project.
Quality and Compliance
VivaBioCell continuously strives to implement a certified quality management system to ensure the quality of our products and services and is certified ISO 9001:2015 by Kiwa, a world top 20 leader in testing, inspection and certification.

