ATMP for Osteoarthritis Using AD-MSCs
VivaBioCell developed a cost model able to simulate the cost of cell therapy production on the basis of a real case study, the ADIPOA clinical trial manufacturing process performed at the Center for Cell Manufacturing Ireland (CCMI) GMP-facility (Galway, Ireland).
The ADIPOA clinical study envisaged the intra-articular administration of autologous Adipose tissue derived-Mesenchymal Stromal Cells (AD-MSCs) for the treatment of advanced Osteoarthritis (OA) and demonstrated the safety and the efficacy of this treatment.
Our cost model examined in detail the costs of the CCMI manual manufacturing process with respect to the NANT 001 System automated cell expansion procedure.
Increasing Manufacturing Process Efficiency
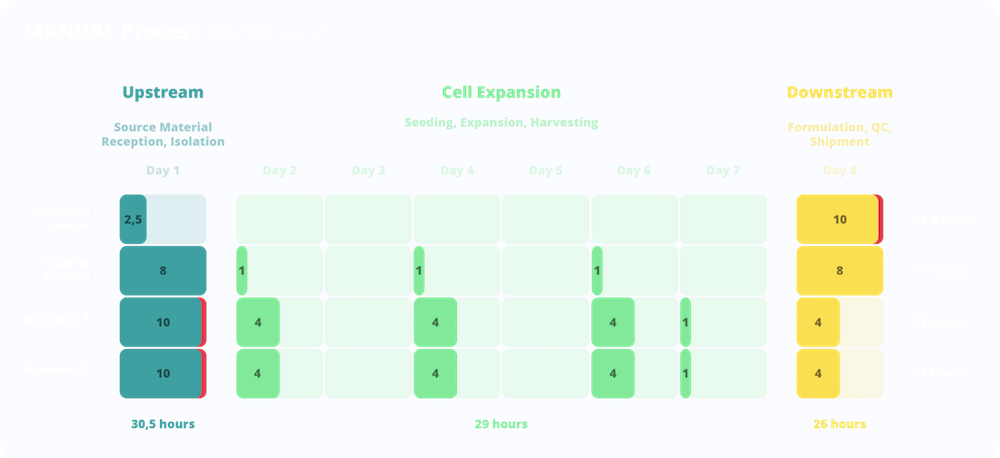
The CCMI manual manufacturing process was broken up into the main operating parameters: the period of use of the production plant (300 days), the daily working time of operators (8 hours), the scheduling of the productions in order to avoid operational overlapping of critical isolation phases (every 6 to 8 days) and the average expansion process duration (8 days).
On the basis of this assumptions, 50 therapeutic doses can be produced by one Grade A/B facility with 96,5 man-hour for one production.
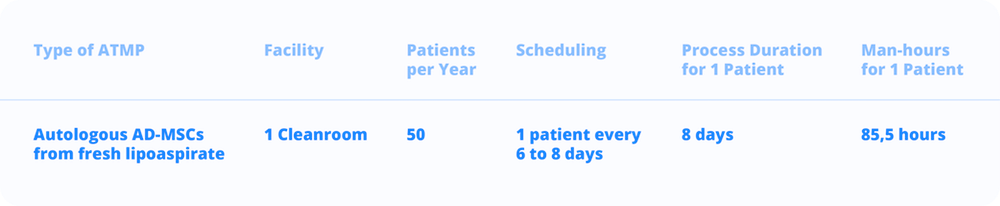
The man working-hours for the manufacturing of one therapeutic dose were calculated considering in detail the contribution of lab Technicians (Operators), QC (Quality Control) and QP (Qualified Person) for each phase (upstream, cell expansion and downstream).
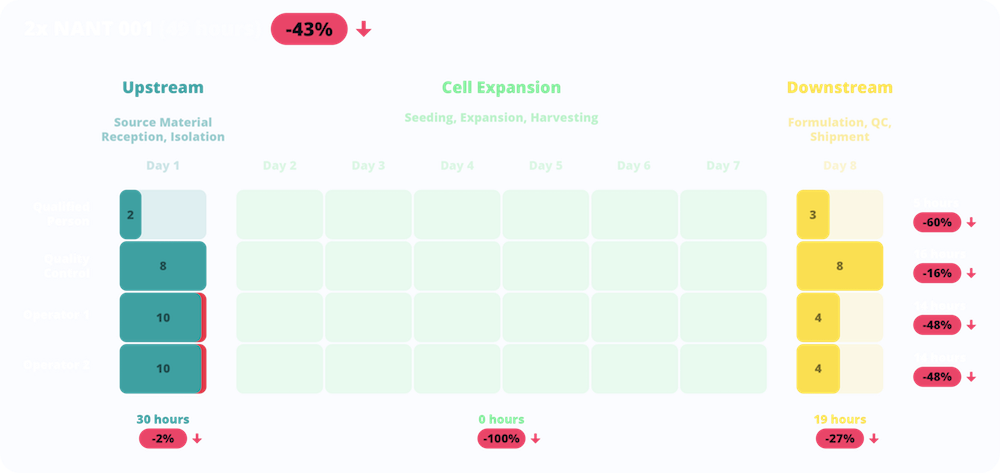
Comparing the manual process with the NANT 001 System, the cost-model demonstrated a dramatic decrease in the number of working hours required by both Lab Technicians (-48%) and by the QC/QP staff. The manual process requires quality control tests during the expansion phases which are not due in the automated NANT 001 system with a consequent reduction of 16% of quality control workload. Moreover, NANT 001 System stores raw data and generates automatically a Cell Culture Report, making process traceable and QP tasks effortless and more efficient.
Costs Reduction
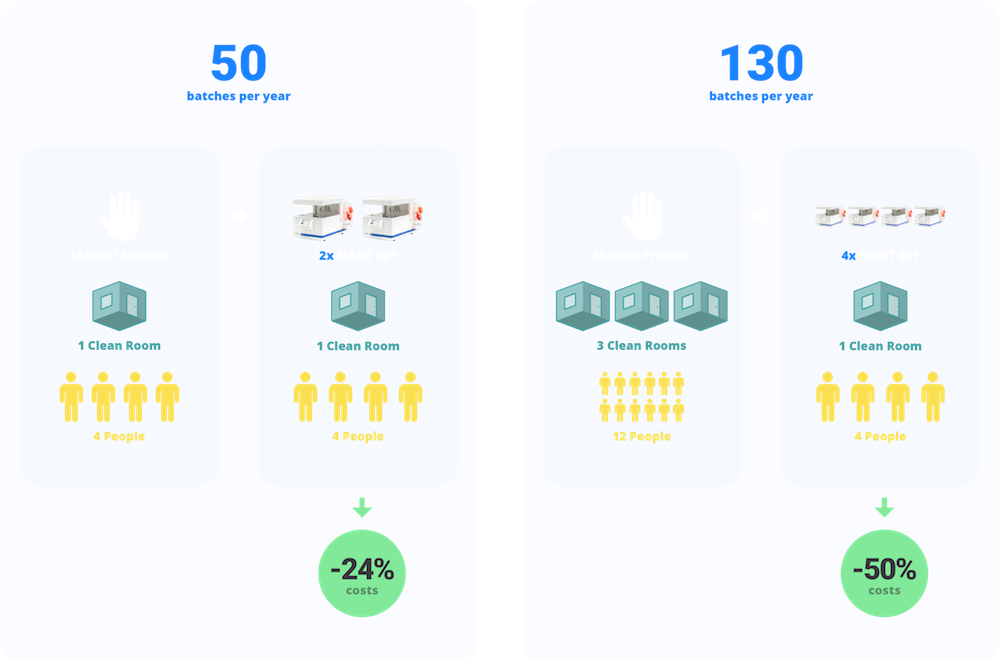
Direct fixed costs (calculated by depreciation of total cleanroom spaces and Capex) and direct variable costs (materials and reagents necessary for cell expansion, Quality Control tests, gowning, cleaning and disinfection of the production plant) were calculated in detail for both the manual and NANT 001 automated cell expansion processes. In a scenario where this case is applied to the same production plant, same personnel and with the same scheduling of productions, the model indicates that the investment in two NANT 001 Systems is sufficient to produce the same amount of 50 therapeutic doses, with a cost reduction of 24% in the cost of each single therapeutic dose.
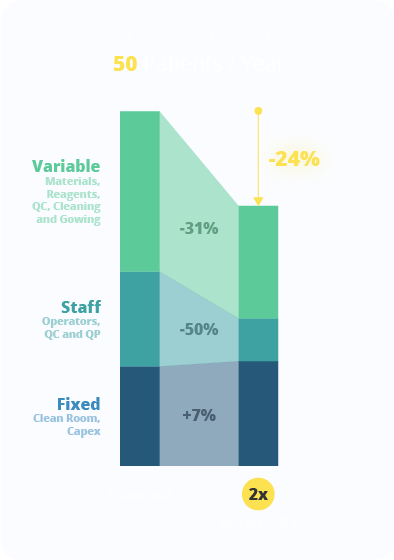
The dramatic decrease in the number of man-working hours has a clear impact on both staff (-43%) and direct variable costs. The access to the cleanroom is due for the upstream and downstream phases only with a consequent cost-reduction in gowning, cleaning and disinfection, while the NANT System allows the optimization in the use of material and reagents.
Gain of Productivity
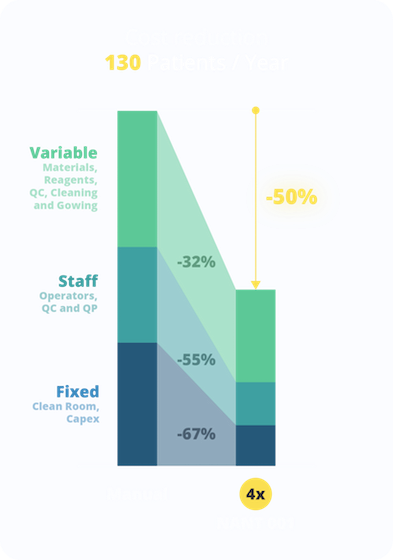
The cost model demonstrates how the automated NANT 001 Systems enable a gain of productivity by the use of 4 bioreactors installed in a Grade D cleanroom: 130 annual therapeutic doses of AD-MSCs can be produced with a concurrent cost-containment of 50% with respect to the manual process, where a triplication of the Grade A/B cleanrooms and of the personnel would instead be needed.