NANT 001 System
An innovative bioreactor system for automated cell therapy bioprocessing, evolved from manual cell culture principles but developed to overcome the challenges linked to traditional open procedures. It has been designed for safe, robust, traceable and cost-effective cell expansion and harvest, to achieve more productive cGMP-compliant manufacturing processes at small-scale.

Integrated Cell Imaging System
Cells are visually monitored in real-time thanks to the integrated liquid lens-based, autofocus-capable microscope, which periodically provides clear and sharp downloadable images while the culture is ongoing.
Automated Cell Confluency Calculation
In adherent and semi-adherent cell culture settings, confluency is automatically calculated, allowing for real-time expansion monitoring. This feature can be used to identify the optimal timeframe for cell harvesting, accommodating intrinsic variability such as that given by autologous starting materials.

Remote Access to Real-Time In-Process Control
In-process control information can be accessed remotely in real-time through the dedicated web-application. This means that users can monitor the culture performance from any device, anywhere - even at their coffee break!

Easy to Use
NANT 001 is a self-contained system with an intuitive Touch Screen that provides you step-by-step instructions, for maximum ease of use and process risk reduction.

Closed System
NANT 001 Cartridge is a sealed, closed-system, GLP biocompatibility-tested, sterile device, designed to prevent cross-contamination. Reagents and waste products are circulated to the dedicated containers by a peristaltic pump and the use of pinch-tube electrovalves.
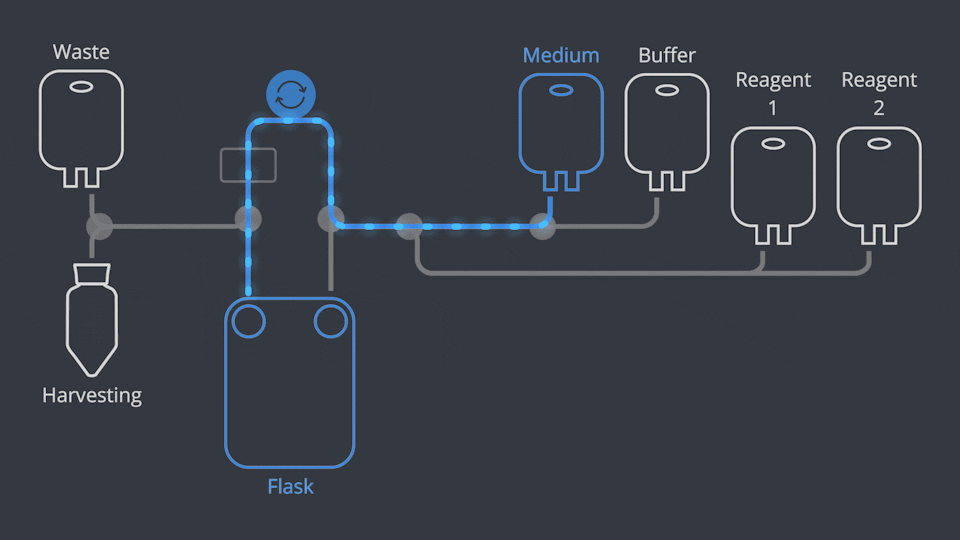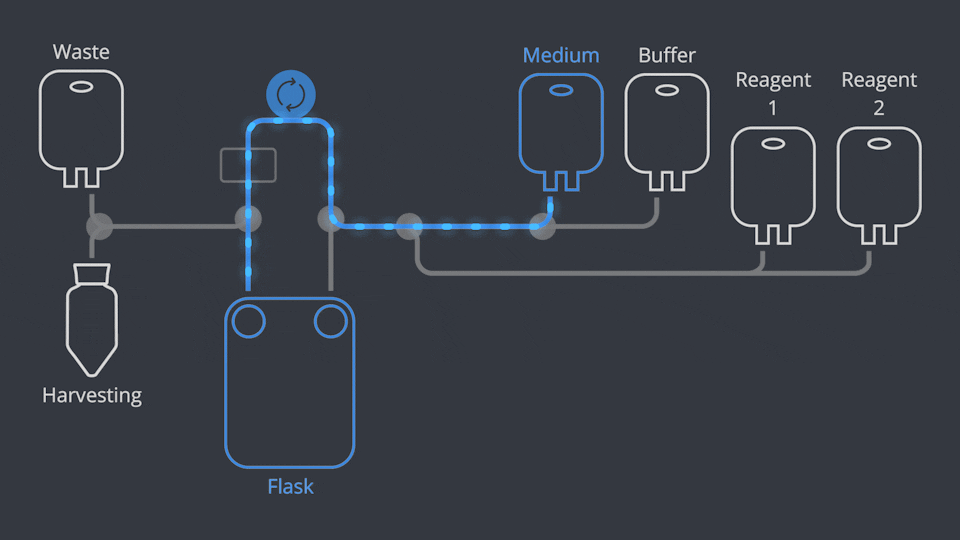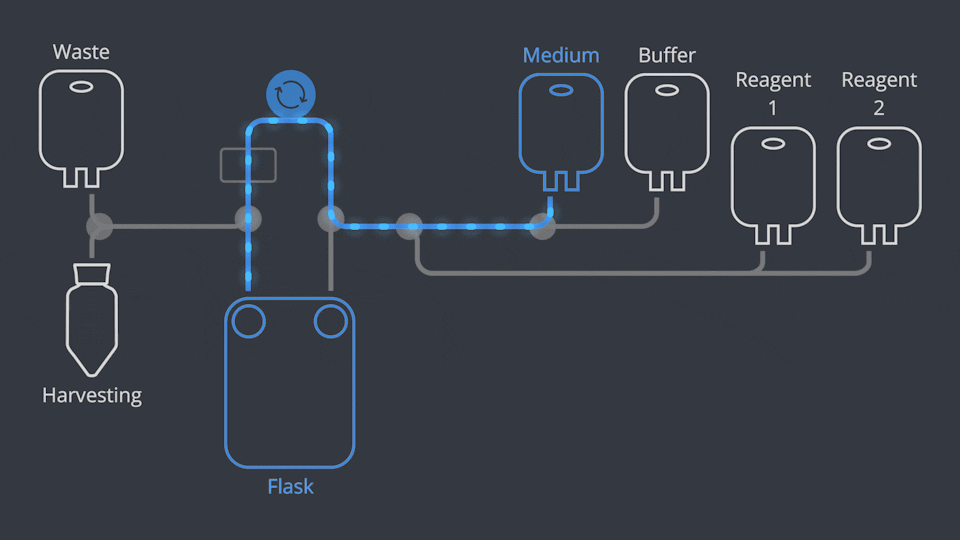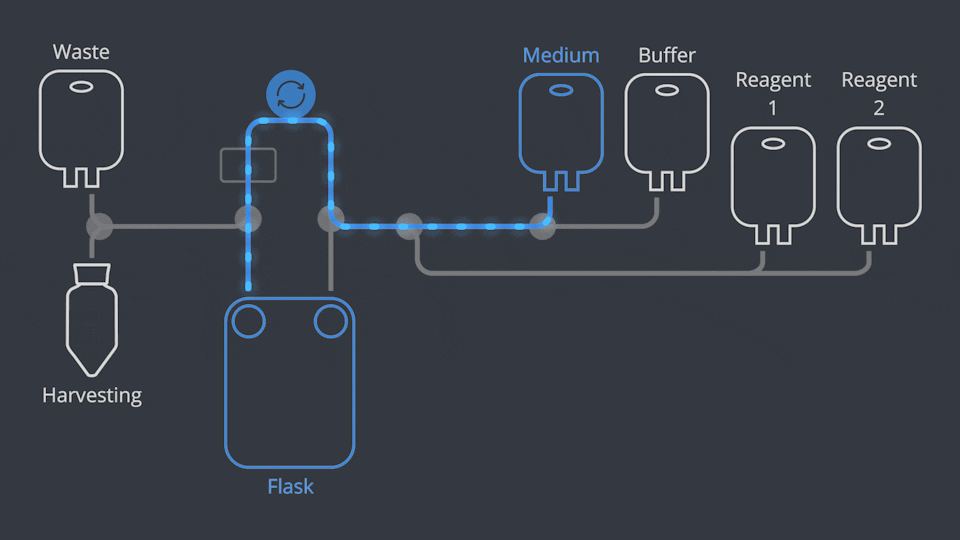
Aseptic Connections, in seconds
As in all NANT Automated Cell Culturing Systems, the different sections of NANT 001 Cartridge are designed to be quickly and easily connected and disconnected - when required - thanks to the integration of validated aseptic connectors and disconnectors.

Automated Tilting and Shaking
The NANT 001 Cartridge flask is moved following gentle and efficient pre-set automated operations, ensuring consistency and reproducibility in even reagent distribution, cell seeding and detachment.
Ready for DSP
NANT 001 System output is provided in the form of a cell suspension, contained into a conical-bottom centrifuge tube which makes your cell material ready for the required downstream processing operations.

Traceability of Disposables and Users
Process traceability is ensured by barcode-based identification of the NANT 001 Cartridge components and of any user actively involved in the operation.

Traceability of Operations
The Cell Culture Report is a fully automated recording of the events occurred during processing with the NANT 001 System. This information, provided in a downloadable .PDF format, can be used to track process compliance and can be easily integrated in any QMS as an auditable document. See example.

Gas Mixing Accessory
NANT G-MIX is the most reliable and flexible choice allowing for a precise flow regulation and mixing of input gasses, designed to be implemented within the NANT 001 System. Each NANT G-MIX can serve up to 5 NANT 001 Systems.

NANT 001 Cartridge
Components you already know, engineered in an easy-to-load, single-use closed-system for safe and cGMP-compliant cell processing.

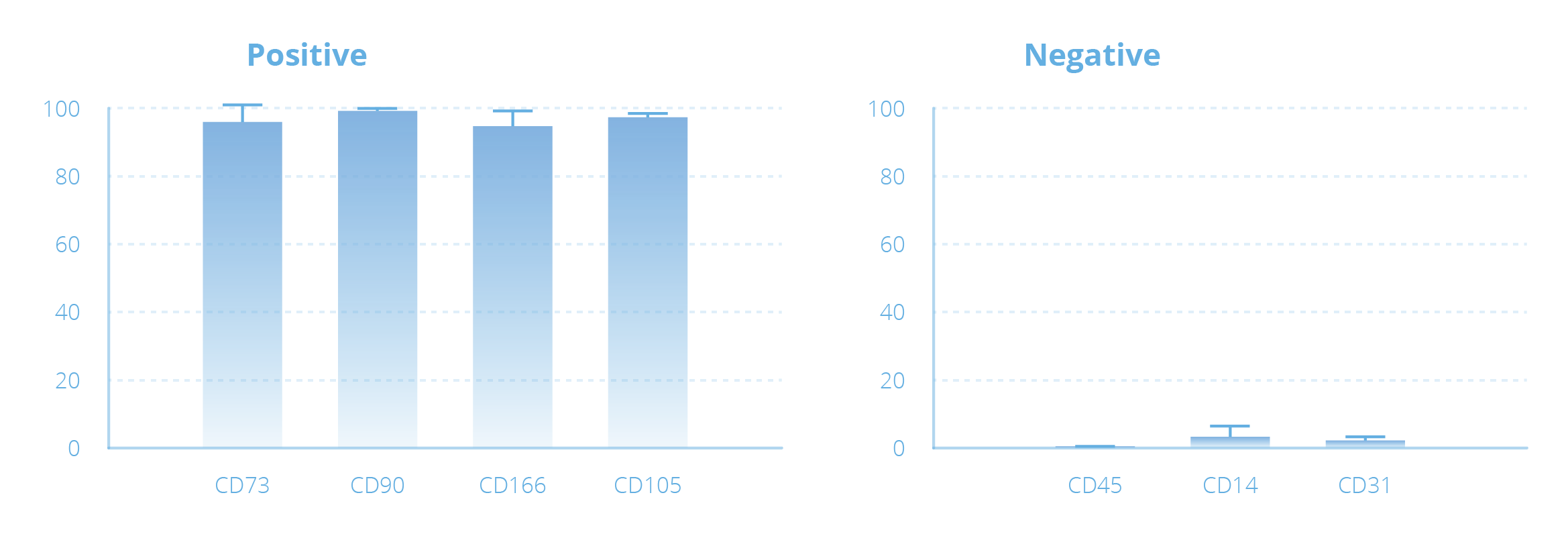
Example of Mesenchymal Stem Cell Expansion with the NANT 001 System
| Amount of starting material | Days of culture | Cell Culture Surface (cm2) | No. of cells harvested | Density of harvested cells (no. of cells/cm2) | Viability of harvested cells (%) |
|---|---|---|---|---|---|
| 10 ml of fresh Adipose Tissue |
10 ± 3 | 636 | 5.37 x 107 ± 1.66 x 107 |
8.98 x 104 ± 2.29 x 104 |
92.6 ± 3.0 |
Representative images of MSCs taken by the NANT 001 System during cell culture

We’re Ready to Support You
The system flexibility and adaptability permit efficient workflow automation for multiple cell-based and cell-derived product manufacturing processes.
Customer-specific cell expansion protocols can be rapidly automated in NANT 001 with ease! Contact us for further information and opportunities.

