
Avvio di uno studio clinico interregionale per TNBC con il farmaco cellulare avanzato aHyC
L'obiettivo generale del progetto è l'avvio di una sperimentazione clinica, a livello interregionale, per il trattamento del carcinoma mammario triplo negativo (TNBC) con vaccino cellulare aHyC. La finalità è di creare la possibilità di fornire terapie antitumorali avanzate personalizzate in un contesto accessibile e conveniente, sviluppando le capacità di innovazione grazie all'introduzione di tecnologie avanzate.
Importo totale: euro 749.059,60
Importo del progetto VivaBioCell: euro 127.064,00
Finanziamento VivaBioCell: euro 0
Zagon medregijskega kliničnega študija za TNBC z naprednim celičnim zdravilom aHyC
Splošni cilj projekta je zagon kliničnega študija na medregijski ravni za zdravljenje trojno negativnega raka dojke (TNBC) s celičnim cepivom aHyC. Namen je ustvariti možnost zagotavljanja naprednih personaliziranih protitumorskih terapij v dostopnem in ugodnem okolju, razvijajoč inovacijske zmogljivosti z uvedbo naprednih tehnologij.
Skupni znesek: euro 749.059,60
Znesek projekta Vivabiocell: euro 127.064,00
Financiranje s strani VivaBioCell: euro 0
Initiation of an interregional clinical study for TNBC with the advanced cellular drug aHyC
The general objective of the project is the initiation of a clinical trial at an inter-regional level for the treatment of triple-negative breast cancer (TNBC) with the aHyC cellular vaccine. The aim is to create the possibility of providing advanced personalized anti-tumor therapies in an accessible and affordable context, developing innovation capabilities through the introduction of advanced technologies.
Total amount: EUR 749,059.60
Amount of the Vivabiocell project: EUR 127,064.00
Funding from VivaBioCell: EUR 0
Partners:
Celica, biomedicinski center, d.o.o.
Onkološki inštitut Ljubljana
VivaBioCell S.p.A.
Università degli studi di Udine
Azienda Sanitaria Universitaria Friuli Centrale
Zavod Republike Slovenije za transfuzijsko medicino
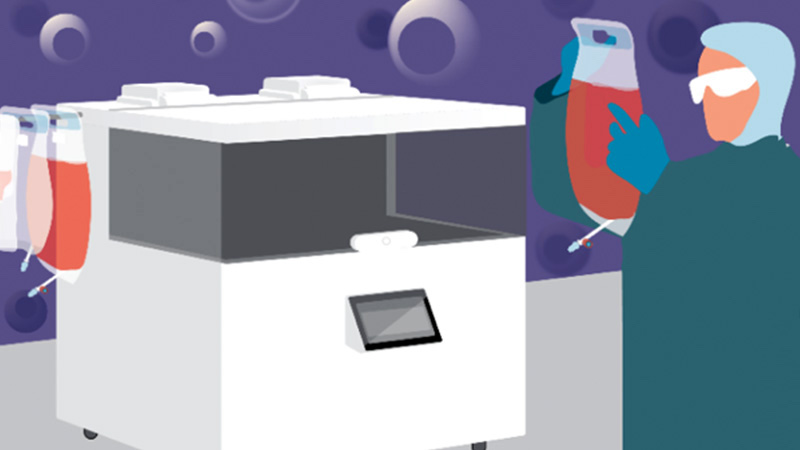
Watch Now the Recording of our Webinar "GMP-Compliant Manufacturing in a Closed-System Automated Bioreactor with a Novel Cell Culture Medium"
In this webinar, VivaBioCell and Fujifilm Irvine Scientific discussed how the utilization of NANT XL System with the Prime-XV MSC X SFM medium can facilitate ATMP manufacturers to meet the compliance with GMP requirements.
In particular, these results of bone marrow-derived MSCs expansions obtained in a cooperation between VivaBioCell, Fujifilm and Educell highlight the advantages of cell therapy manufacturing automation in closed systems in combination with xeno-free cell culture media.
Register for free to watch the webinar.

GMP-Compliant Production of Autologous Adipose-Derived Stromal Cells in the NANT 001 Closed Automated Bioreactor
The results of this GMP-validation study conducted at the Regenerative Medicine Institute (REMEDI) confirm the ability of NANT 001 System in conducting automated MSCs expansions, meeting all the GMP requirements in terms of safety, traceability and reproducibility in a cost-effective manner.
Read the full article "GMP-Compliant Production of Autologous Adipose-Derived Stromal Cells in the NANT 001 Closed Automated Bioreactor".

VivaBioCell is Gold Sponsor of Advanced Therapies Virtual Congress & Expo (London, 5-6 October 2021)
We're thrilled to confirm our participation at the next Advanced Therapies Congress & Expo (London, 5-6 October 2021) as GOLD sponsor of this international event.
Come and meet our Team at Booth No. 13 where you will have the opportunity to know more about our automated bioreactor Systems NANT 001 and NANT XL.
And make sure to attend the lecture by Prof. Frank Barry (5 October at 5.00 pm) to get some insights on the results of a GMP Validation study conducted at the Regenerative Medicine Institute (Galway, Ireland) with our NANT 001 System.

Join us for our new webinar on September 9th 2021 (3.00 pm - CET)
We are delighted to announce the free webinar "Overcoming the challenges of ATMP manufacturing with novel automated systems for cell expansion: Tackling GMP-compliance, scalability and cost efficiency" where we will discuss the challenges of cell therapy manufacturing and how VivaBioCell automated technology helps overcome them.
The webinar will also feature a presentation by Aoife Duffy, who will provide the feedback and point of view of our partner HiTech Health.
Join us on September 9th 2021 at 3.00 pm CET via free registration.

VivaBioCell at the "European Orthopaedic Research Society" Conference
VivaBioCell will attend next EORS Annual Meeting in Rome (15-17 September 2021) and showcase novel innovative technologies revolutionizing the approach to traditional orthopedics.
Come visit our booth and have a chat with our team!

FDA Authorizes ImmunityBio to Conduct a Trial of its First-in-Human, Cryopreserved, Memory Cytokine-Enriched NK Cell (m-ceNK) Platform in Solid Tumors
ImmunityBio, Inc. announced it has received FDA authorization to conduct a Phase 1 study to evaluate the safety and preliminary efficacy of its m-ceNK platform in subjects with locally advanced or metastatic solid tumors.
The cell therapy product will be manufactured thanks to a novel method, which yields multiple clinical-doses from a single apheresis using the company's proprietary NANT 001 Bioreactor, developed and supplied by VivaBioCell.
Read ImmunityBio Press Release: FDA Authorizes ImmunityBio to Conduct a Trial of its First-in-Human, Cryopreserved, Memory Cytokine-Enriched NK Cell (m-ceNK) Platform in Solid Tumors.

Scaling-up the production of BM.allo-MSC with NANT 001 System to treat severe COVID-19 patients
Recently, our partner NantKwest, Inc. has announced the FDA authorization of an IND Application for a Mesenchymal Stem Cell Product aimed at the treatment of COVID-19 patients that require ventilation.
In this ongoing Phase 1b, randomized, double-blind, placebo-controlled study (NCT04397796), patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 are currently being treated with BM-Allo.MSC, an allogeneic Mesenchymal Stem Cell (MSC) product derived from human Bone Marrow that is produced with the NANT 001 System, a technology that is truly enabling a scalable manufacture of this therapy for its broadly distribution.
"There is an urgent need to develop broadly accessible treatment options for the devastating outcomes seen in the thousands of COVID-19 patients who are facing severe disease with ARDS and 'cytopathic storm,'" said Patrick Soon-Shiong, M.D., Chairman and Chief Executive Officer of NantKwest and ImmunityBio. "While MSC-derived treatments have shown promise in treating patients with ARDS, including those with COVID-19, the ability to scale production to support the overwhelming patient need has been a challenge. Our proprietary GMP-in-a-Box enables the rapid and scalable manufacture of our fully human BM-Allo.MSC product, overcoming this previous limitation to advance a promising new treatment to those patients who are most in need. Due to our proprietary methods, we are well positioned to rapidly advance BM-Allo.MSC during the current wave of COVID-19, with an anticipated trial initiation in Q2".
The therapeutic will be administered to a total of 45 patients in the critical care or ICU setting. The primary objectives of the study include overall safety and reduction in time on ventilator. The secondary objective will focus on the efficacy of BM-Allo.MSC in reducing the number of days patients require oxygen, duration of hospitalization, and mortality.

VivaBioCell is now a partner of the Centre for Advanced Medical Products (CAMP) - Sweden
We're very proud to be the first international partner of the Centre for Advanced Medical Products (CAMP), making our technology available to develop new innovative therapeutic applications in Sweden.
We will closely cooperate with the Lead Partner Region Örebro Län, within a project aimed at designing and implementing new therapies based on expanded MSCs for the treatment of osteoarthritis, that will envisage the use of NANT Systems for the manufacturing of these ATMPs.

Automated GMP cell expansion: Manufacturing solutions for sustainable therapeutic applications - A webinar by VivaBioCell and Prof. Frank Barry (Scientific Director, Regenerative Medicine Institute, Galway – Ireland)
You can now watch the full recording of VivaBioCell webinar Automated GMP cell expansion: Manufacturing solutions for sustainable therapeutic applications, providing insights on how NANT 001 and NANT XL Systems can improve GMP-compliant automated cell therapy manufacturing, paving the way towards the sustainable and cost-effective implementation of Advanced Therapies in the clinical practice.

VivaBioCell at EuroBioHighTech 2020 Conference
Thanks for visiting us at our booth during the EuroBioHighTech 2020 conference! We have been delighted to be part of this event, with so many networking opportunities!
It was a pleasure also for us to present the advancements done within the Interreg A.R.T.E. Project, where we have proven that a close cooperation between the different partners is making Advanced Therapies a reality and improving patients' lives.

VivaBioCell is the Silver Sponsor of Advanced Therapies Virtual Congress & Expo (8–11 September 2020)
We are attending the Advanced Therapies Congress, enjoying our first virtual conference and looking forward to e-meet new partners with the common vision of making Advanced Therapies the new standard of clinical care.
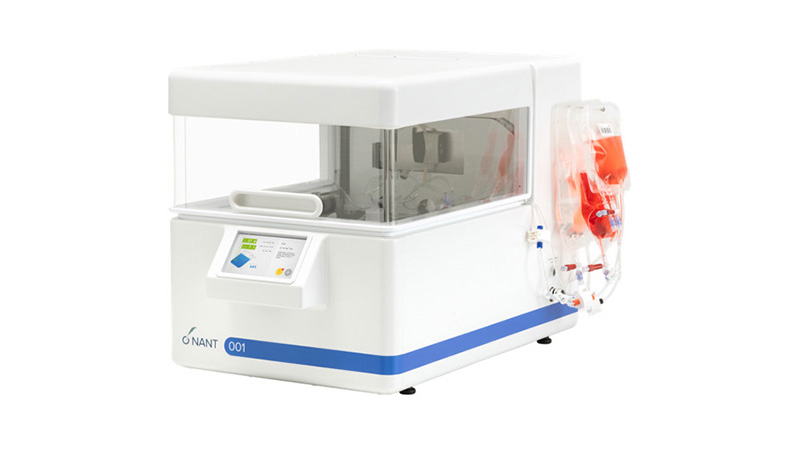
The White Paper "Automated Expansion of Adipose-Derived Mesenchymal Stem Cells (AD-MSCs) with NANT 001 System" is now available
The White Paper "Automated Expansion of Adipose-Derived Mesenchymal Stem Cells (AD-MSCs) with NANT 001 System" describes a GMP-compliant process to automatically expand AD-MSCs isolated from fresh adipose tissue using the NANT 001 System, and the results obtained in comparative experiments where the yield, viability and MSCs characterization have been evaluated.
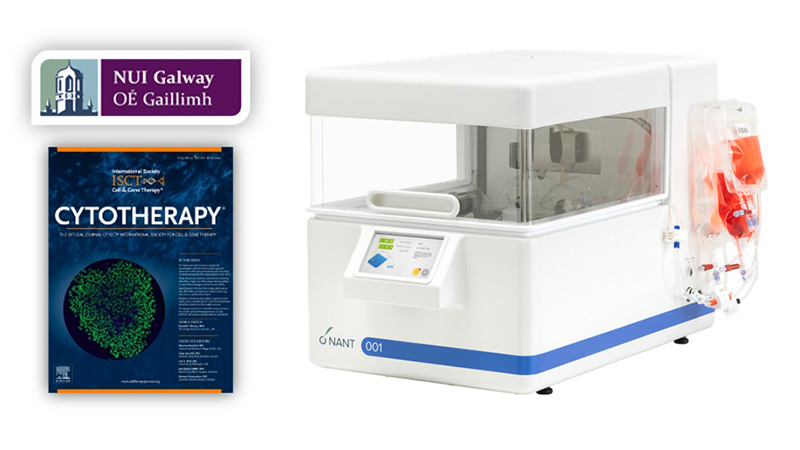
Validation of the NANT 001 Bioreactor System for automated adipose-derived MSCs expansion
The results just published by The Regenerative Medicine Institute (REMEDI, Galway, Ireland) show how the NANT 001 System is able to effectively expand autologous MSCs derived from adipose tissue, in aseptic conditions and with a standardized automated process, paving the way for the utilization of VivaBioCell technology in the manufacturing of cell therapy products for regenerative medicine applications.
Read the full story published on "Cytotherapy" ("Validation of the NANT 001 Bioreactor System for automated adipose-derived mesenchymal stem cell expansion"), the official journal of International Society for Cell & Gene Therapy (ISCT).

Immuno-Cluster
VivaBioCell is a beneficiary of Immuno-Cluster, a project approved under the Interreg V-A Italy-Slovenia 2014-2020. This Project aims to create a cross-border collaboration among Italian and Slovenian actors and to develop a clinical protocol for the treatment of triple negative breast cancer.
The vaccine obtained from autologous immune cells (cellular immuno-hybridoma) will be produced both by manual methods and also by the NANT 001 System in order to make the therapy affordable to an increasing number of patients.

See You in September!
Due to the current COVID-19 outbreak both Advanced Therapies Congress & Expo and 19th ESSKA Congress will be postponed in September 2020.
Check out the new dates, and follow-us on our website and Linkedin where we will keep you posted with updates regarding these events.

VivaBioCell is proud to be attending the event "Meet in Italy for Life Sciences 2019" in Trieste next 16-19th October
Come visit us at our booth and make sure to join our workshop taking place on 17th October at 9:30 (Sala 3) where we will shed lights on the A.R.T.E. Project (Advanced Regenerative Therapies Ecosystem), and present the operative approach guiding the cooperation of public and private entities with the final aim of improving patients' quality of life, containing the healthcare expenditure, and creating business opportunities for both companies and public research institutions.
Visit https://meetinitalylifesciences.eu/ for more information.

VivaBioCell attending the "2nd Annual Workshop on GMP Manufacturing, Scale-up and Challenges of ATMPs"
We are happy to confirm our participation at next Annual Workshop on GMP Manufacturing, Scale-up and Challenges of ATMPs which will be held in Galway (Ireland) on 13-14th November 2019. This event will provide deep insights about manufacturing challenges and possible applications scenarios of cell-therapies, ranging from MSCs, CAR-T and NK cells.
Both our NANT 001 and NANT XL Automated Cell Culture Systems will be showcased during the conference, and a specific workshop where we will describe how our solutions can overcome the current hurdles of cell-therapy manufacturing will take place on Thursday 14th November.

The NANT family of automated cell culture solutions will be showcased at the ASCO Meeting, Chicago, IL (May 31 - June 3, 2019)
Meet us at ASCO Meeting 2019, Booth 24080 (NANT) to be the first to know more about NANT XL System, the evolution of automated cell expansion systems for mid- up to large-scale batch sizes. The NANT XL System features subculturing capability - fully automated and in a closed system! - as well as other key features common to NANT 001 System, such as integrated microscopic imaging, remote monitoring, and more.
VivaBioCell is part of NantCell, Inc., a subsidiary of Nantworks, LLC. NANT is focused on solving humanity's greatest problems, transforming how we work, live and play. NANT is the umbrella organization delivering on the next age of the fourth Industrial revolution, converging breakthroughs in biology, healthcare, energy, communications, and artificial intelligence. Outcomes driven, each of our NANT companies are expanding the boundaries of science and technology for actionable, real world applications today.

VivaBioCell at BioSpine 7th International Congress on Biotechnologies for Spinal Surgery
As industrial sponsor, VivaBioCell will participate to the BioSpine 7th International Congress on Biotechnologies for Spinal Surgery which will take place in Rome at the Auditorium Antonianum from 3rd to 5th April 2019.
On Friday the 5th at 12.30 am we will illustrate how the NANT 001 System enables Stem Cells therapies for disc regeneration to become affordable and scalable. The NANT 001 System will also be available for display at stand number 10. We are looking forward to meet you!

NANT 001 System at TERMIS-European Chapter Meeting 2019
We are pleased to announce that the Regenerative Medicine Institute (REMEDI) of Galway, Ireland, will present the amazing results obtained on the Validation of the NANT 001 Bioreactor System for Automated Adipose-Derived Mesenchymal Stem Cell Expansion.
Don't miss the podium presentation at TERMIS-EU 2019.

PREFER (sviluppo di un PRodotto biocompatibile per la tErapia delle FERite difficili)
PREFER (sviluppo di un PRodotto biocompatibile per la tErapia delle FERite difficili), è un progetto finanziato dalla Regione Autonoma Friuli Venezia Giulia nell'ambito del POR-FESR 2014-2020, attività 1.3b, che ha lo scopo di sviluppare un prodotto innovativo basato sulla combinazione di una matrice extracellulare, cellule endoteliali autologhe e fattori di crescita al fine di consentire la rivascolarizzare, la ricrescita dell'epitelio cutaneo di tessuti colpiti da ferite difficili (quali piede diabetico, ulcere) e di evitarne l'amputazione. Dopo aver opportunamente valutato l'efficacia della terapia da un punto di vista istologico e funzionale attraverso studi in vitro e in vivo, lo scopo finale del progetto sarà ottenere un prodotto ATMP combinato che possa essere testato clinicamente mediante studi di fase I/II. Al termine della sperimentazione clinica, prevista successivamente alla chiusura del progetto, si prevede che il prodotto che possa esser commercializzato ad un costo contenuto grazie all'espansione delle cellule endoteliali mediante la tecnologia NANT.
I Partner di progetto sono Zeta Research, il Dipartimento di Scienze delle Vita dell'Università di Trieste , il Gruppo di Biologia Cardiovascolare dell'International Centre for Genetic Engineering and Biotechnology (ICGEB), con la collaborazione dell'Azienda Sanitaria Universitaria Integrata di Trieste e Azienda Sanitaria Universitaria Integrata di Udine.
Il contributo concesso è di 718.486,92 € per un budget totale di 1.102.085,00.

VivaBioCell at ISCT EU 2018 Regional Meeting
Meet us at ISCT EU 2018 Regional Meeting, held in Florence, Italy on September 12-14, 2018! Come to booth #12 and talk to us to understand how the NANT 001 System can be integrated into your process.

ARTE Project - Interreg V-A Italy-Slovenia 2014-2020
VivaBioCell is a beneficiary of A.R.T.E. (Advanced Regenerative Therapies Ecosystem), a project approved under the Interreg V-A Italy-Slovenia 2014-2020.
The common challenge of the ARTE Project within the EU-Financed INTERREG V-A Italy Slovenia Programme, is to contribute to make the cross-border area become a Reference Point in the European ecosystem for innovative therapies and regenerative medicine. Regenerative Medicine is a strategic priority in the Horizon 2020 Program financed by the European Commission.
The General Objective of ARTE is to strengthen the cooperation among Italian and Slovenian actors such as hospitals and clinics, research centres, SMEs, Technology Parks and private investors in this key sector.
ARTE is formally endorsed by the Central Directorate for Health - Regione Autonoma Friuli Venezia Giulia, Italy and the Nacionalni inštitut za za javno zdravje - National Institute of Public Health, Slovenia.
ARTE will focus on a nearly-commercial innovative therapy for OA (Osteoarthrosis). The Sponsors have completed pre-clinical and clinical studies, and over a period of 4 years, have treated above 900 patients with excellent safety and efficacy, utilizing undifferentiated, expanded, MSC (Mesenchymal Stem Cells) collected from Bone Marrow. Several scientific papers have been published in peer-reviewed journals.
The Project Partners are also very interested to implement other therapies (e.g. using MSC harvested from Adipose tissue) that are near commercialization. Particularly those developed with the support of H2020 and other programmes of the EC, in line with the institutional objective of the Commission “to create multi-disciplinary research teams with a "European" stamp in order to attain the indispensable size known to experts as critical mass".
The Scope of ARTE includes activities such as : clinical trials (Italy and Slovenia); collaboration with 3 research centers / hospitals; transfer and training of clinical and biological protocols; involvement of 2 biomedical networks; adoption of an automated mini-bioreactor (Project Partner VivaBioCell Italy) to help make the novel OA therapy affordable, standardized and scalable, reaching a much higher number of patients; involvement of SMEs for the further development of innovative products / services.
The final results in the Programme area are: a) to prime entrepreneurial growth in the fast-growing global market for regenerative medicine and b) to help innovative therapies that have proven safety and efficacy become much more widespread and available at leading hospitals in Slovenia and Italy.
Project partners are:
LP-VP - VivaBioCell S.p.A.
PP2 - Tehnološki park Ljubljana d.o.o.
PP3 - Ortopedska bolnišnica Valdoltra
PP4 - Univerza v Ljubljani (Fakulteta za farmacijo)
PP5 - Azienda Sanitaria Universitaria Integrata di Udine
PP6 - BioValley Investments

VivaBioCell at World Advanced Therapy and Regenerative Medicine Congress 2018
Come and meet us by Stand 42 at The World Advanced Therapy and Regenerative Medicine Congress 2018 (London, 16-18 May), to learn more on how VivaBioCell can support you in developing small- to mid-scale batch size products with innovative automated solutions for cell-based and and cell-derived product manufacturing!

VivaBioCell Webinar on Cell and Gene Therapy Insights
On 8th March 2018 (07:00 PST; 10:00 EST; 15:00 GMT; 16:00 CET) VivaBioCell will be presenting a live webinar about "Meeting scalability challenges for autologous cell-based manufacturing with automated closed-system solutions", which will be hosted by Cell and Gene Therapy Insight, a platform designed to meet the needs of all stakeholders across the global cell and gene therapy community. Go to the event page to learn more and register for free!

Meet VivaBioCell at the CIBIO Career Day - University of Trento
We are glad to meet talented biotechnology students for career opportunities - and to present our mission and vision in the context of the interface between biology, technology and market - at the CIBIO (Center for Integrative Biology) Career Day, University of Trento, on March the 14th 2018 (1.15pm: company presentation; 2-6pm: interviews with students and careers opportunity discussion).

Meet us at EURO BIO HIGH TECH 2017 forum in Trieste!
VivaBioCell will participate to the Euro Bio High Tech 2017 forum, the most important event devoted to the biomedical sector, biotechnology and bioinformatics in the Alpe Adria area, which will take place in Trieste, at the Palazzo dei Congressi in the Maritime Station, from 21 to 22 September 2017.
On Thursday the 21st, at 10.30, we will illustrate the SISICOR project, as well as the NANT 001 bioreactor system, which will be available for display at our stand. We are looking forward to meet all interested subjects!

NANT 001 at ESGCT
We are happy our abstract “A novel automated, closed system solution for simple, de-risked and cost-effective autologous ATMP manufacturing: the NANT 001 bioreactor” has been selected for a poster presentation at ESGCT and DG-GT Collaborative Congress in Berlin, during the session on Thursday 19th October.
Come and talk to us if you're attending and would like to learn more about VivaBioCell and our technology!

VivaBioCell at PARIS REGMED 2017
VivaBioCell is proud to announce that it will attend PARIS REGMED 2017, the 1st European matchmaking event in the field of regenerative medicine – held in Paris next week on 7th July.
We will give a short presentation on our NANT 001 bioreactor system during the afternoon session: “Presentation of key International ecosystems in the field: presentation of clusters and regenerative medicine stakeholders”.

NANT 001 at The World Advanced Therapy and Regenerative Medicine Congress
VivaBioCell will present its novel NANT 001 bioreactor system with the talk: “Automated Systems for Autologous ATMP Manufacturing: Quality Considerations” at 16:20 on day 2 of The World Advanced Therapy and Regenerative Medicine Congress, organised by Terrapin and held in London from 16th to 18th May 2017.
Come and talk to us if you're attending and would like to learn more about VivaBioCell!

POR FESR 2014-2020 FVG: OPPORTUNITA' PER UNA CRESCITA SOSTENIBILE.
VivaBioCell is a beneficiary of SISICOR (Sistema Implantare per la Stabilità immediata di impianto in Chirurgia Orale di Ricostruzione), a project approved under the POR FESR 2014-2020 Friuli Venezia Giulia.
SISICOR: nuovo Sistema Implantare per la Stabilità immediata di impianto in Chirurgia Orale di Ricostruzione
Contributo finanziato € 252.856,15 (DGR n. 849/2016. Pratica n. 24140/2016)
Data di avvio: 01 Febbraio 2017. Data prevista di conclusione: 31 Maggio 2018.
Descrizione. Il progetto "SISICOR" intende intraprendere una complessa attività, finalizzata ad un radicale avanzamento degli attuali sistemi implantari e protesici. Verranno ricercate "nuove soluzioni di implantologia e protesica dentale, in abbinamento a scaffold (strutture) in grado di accrescere la proliferazione delle cellule staminali per la rigenerazione del tessuto osseo maxillofacciale" per soddisfare le diverse esigenze cliniche (es. completa edentulia, condizioni post estrattive, traumatologia) con attenzione agli aspetti clinici del processo pre e post implantare, alle procedure chirurgiche digitali e di produzione personalizzata 3D.
Obiettivo. L'obiettivo finale è quello di identificare delle soluzioni integrate (scaffold + sistemi di ancoraggio + sistemi protesici) capaci di garantire un'ottima interazione tra l'osso e l'impianto e tra i tessuti molli ed il moncone protesico, riducendo al minimo il rischio di tasche perimplantari profonde ed accorciando notevolmente i tempi necessari al ripristino delle normali funzioni. In particolare si vogliono sviluppare degli impianti innovativi che consentano una procedura chirurgica "a carico immediato" a prescindere dalla qualità dell'osso ove sono posti. Inoltre si intende sviluppare una metodologia innovativa per favorire l'accrescimento osseo e ripristinare la qualità necessaria alla stabilità dell'impianto.
Risultati. Al termine dell'intervento ci si aspetta di ottenere soluzioni per nuovi prodotti di implantologia e protesica che permettano all'utilizzatore finale di affrontare e di risolvere in modo efficace delle problematiche chirurgiche importanti quali: l'applicazione di impianti "a carico immediato"; il recupero di situazioni post-estrattive gravi; il recupero di situazioni traumatologiche importanti. Ci si propone di fornire degli strumenti flessibili e che permettano al chirurgo di affrontare in modo immediato ed efficace diverse tipologie di intervento, incrementando la percentuale di successo e riducendo lo stress sul paziente.

Los Angeles, California – June 22, 2015
NANTCELL acquires VivaBioCell to develop automated “GMP-in-a-box” for next generation large scale cell culture and tissue engineering.
NantCell, a subsidiary of NantWorks, LLC, a company focused on the discovery and development of disease treatments through innovative, cell- based therapies at the molecular level, announced today that VBC Holdings, LLC, a wholly owned subsidiary of NantWorks, has completed the acquisition of privately held VivaBioCell SpA, a leading biotechnology company, located in Udine Northern Italy. Terms of the transaction were not disclosed.
“With the acquisition of VivaBioCell, we have added novel and complementary cell culture capabilities and extended our geographic footprint,” said Dr. Patrick Soon-Shiong, founder of NantCell. “For nearly a decade, the team at VivaBioCell has been focused on discovering and developing therapies that utilize stem cell and tissue engineering for regenerative medicine. Bringing the two organizations together strengthens NantCell's capabilities in tissue engineering and cell culture capabilities.”
“We are thrilled to be combining forces with NantCell,” said Prof. Francesco Curcio, President and CSO of VivaBioCell. “I have collaborated with Dr. Soon-Shiong for over 20 years in the field of stem cell research and integrating with NantCell is the culmination of our many years working together in this field. The mission, culture and leadership team of both companies blend well, creating an ideal atmosphere for collaboration and continued scientific discovery.”
VivaBioCell's automated “GMP-in-a-Box” medical devices enable safe, efficacious, standardized and affordable medical treatments by creating a next generation tissue engineering manufacturing capability. Current applications include developing stem cell for the treatment of osteoarthritis as well as bone regeneration for dental /maxillofacial reconstruction. This automated “GMP-in-a-Box” system will be utilized to develop next generation manufacturing for immune cells utilized in the treatment of cancer such as natural killer cells.
As part of the transaction, NantCell also acquired VivaBioCell's diagnostic product, capable of identifying patients with systemic sclerosis and systemic lupus erythematosus.

